History

KETOTOP® has been invented by Amore Pacific Pharm. in 1995 for the patients with Arthritis or Rheumatic Inflammation who has suffered from the side effects of oral medication of Arthritis including gastrointestinal hemorrhage, Edema, rise of blood pressure, and so on. It has 15 patents by 'Technology of Trans-dermal Drug Delivery System' in the countries such as U.S., U.K., Germany, France, China, Japan, Canada, Italy, Spain, Switzerland, Vietnam, Malaysia, Indonesia, Singapore, and Korea. This technology was very superior at that time and even now, and it is still big hit item by Ketoprofen, fast-acting drug, of which its excellent effects on pain relief and treatment of arthritis. Now is still producing a same plaster by HANDOK after the acquirement of Amore Pacific Pharm factory.
Product Information
[New KETOTOP® Plaster]
- 30mg Ketoprofen Plaster
- 40 sheets of Ketoprofen plaster
- Size of Plaster Sheet
- 10.30 x 6.80 (W x H, cm)
- Expiration Date: Oct-2024 or Better
- Manufactured by HANDOK, Co., Ltd.
Features
- World's First Pain Relief Patch for Arthritis
- Korea No.1 Top Selling Pain Relief Patch for Arthritis & Rheumatic Inflammation
- Ketoprofen, proved the marvelous pain relief effects by clinical trials
- 12 hours Effect
- Over-the-Counter Drug
Active Ingredient

30mg of Ketoprofen for transdermal application
Dosage and Administration
put 1 sheet of KETOTOP® to the affected part twice daily
Indication and Usage

KETOTOP® is indicated for the treatment of signs and symptoms of the following
- Arthritis (Rheumatoid Arthritis)
- Peri-arthritis & Painful or Stiff Shoulder
- Peri-tendinitis & Tendinitis
- Muscular Pain
- Pain/Swelling caused by Trauma (Contusion, distortion, etc)
Storage

Close a zip tightly and then store at ambient (25˚C). Please Avoid UV or Direct Light.
Precautions
1.This product should not be administered to the following patients.
1) Patients who ever showed hypersensitivity to this product or any ingredient of this product.
2) Patients with aspirin asthma (asthmatic attack by non-steroidal analgesic or anti-inflammatory drug) or the history of the disease (They may have asthmatic attack).
2.This product should be carefully administered to the following patients.
1) Patients with bronchial asthma (They may have asthmatic attack).
2) Patients under medical treatment.
3.Adverse reactions
1) In rare cases, anaphylactic symptoms (hives, dyspnea, facial edema, etc) may be occurred. Therefore, if such symptoms occur, stop use of this product.
2) Asthmatic attack may be occurred. If early symptoms, such as rhonchus, stridulous, or dyspnea, is occurred, immediately stop using this product. Such asthmatic attack may be occurred in a few hours after administration of this product.
3) Skin: Pigmentation, xeroderma, and contact dermatitis, such as flare, eruptive, itch, bleb, maceration, irritation, and swelling, may be occurred. In addition, the direct ray of light (ultraviolet ray) may cause photosensitization disease and the whole body may have rashes. Stop use of this product, if such symptoms are severe.
4.General precautions
1) Use of analgesic? Anti-inflammatory drug is not a causal treatment, but a symptomatic therapy.
2) This product may cause in apparent skin infection. Therefore, if used for treatment of inflammation caused by infection, be sure to use appropriate antibiotics or anti-fungal agents at the same time and to observe carefully if there are any adverse reactions.
3) If this product is used for treatment of chronic diseases such as degenerative arthritis (osteoarthritis), consider other treatment method rather than a drug therapy. In addition, be sure to observe carefully the patients if there are any adverse reactions.
4) If there is no improvement when used for about 1 week, stop using this product and consult the doctor or pharmacist.
5.Pregnant or nursing women
1) When this product was orally administered to rats at the late stage of pregnancy, contraction of fetal arterial blood vessel was reported.
2) Administration of this product at the late stage of pregnancy (oral, injection, or rectal infusion) was reported to result in continuous fetal circulation and fetal renal insufficiency.
3) Safety in pregnant and nursing women has not been established. So, massive or broad use of this product for long-term period should be avoided for pregnant, gestational, and nursing women.
6.Pediatric use
Safety in premature babies, newborn babies, infants, and young children has not been established (only a few clinical cases).
7.Warning
1) Do not use this product to eyes or mucous membrane.
2) Administration of this product to damaged skin, mucous membrane, eczema, or eruption may result in temporary irritation or numbing.
3) Do not use this product for treatment of athlete's foot or tinea.
4) Do not use this product in the occlusive dressing manner.
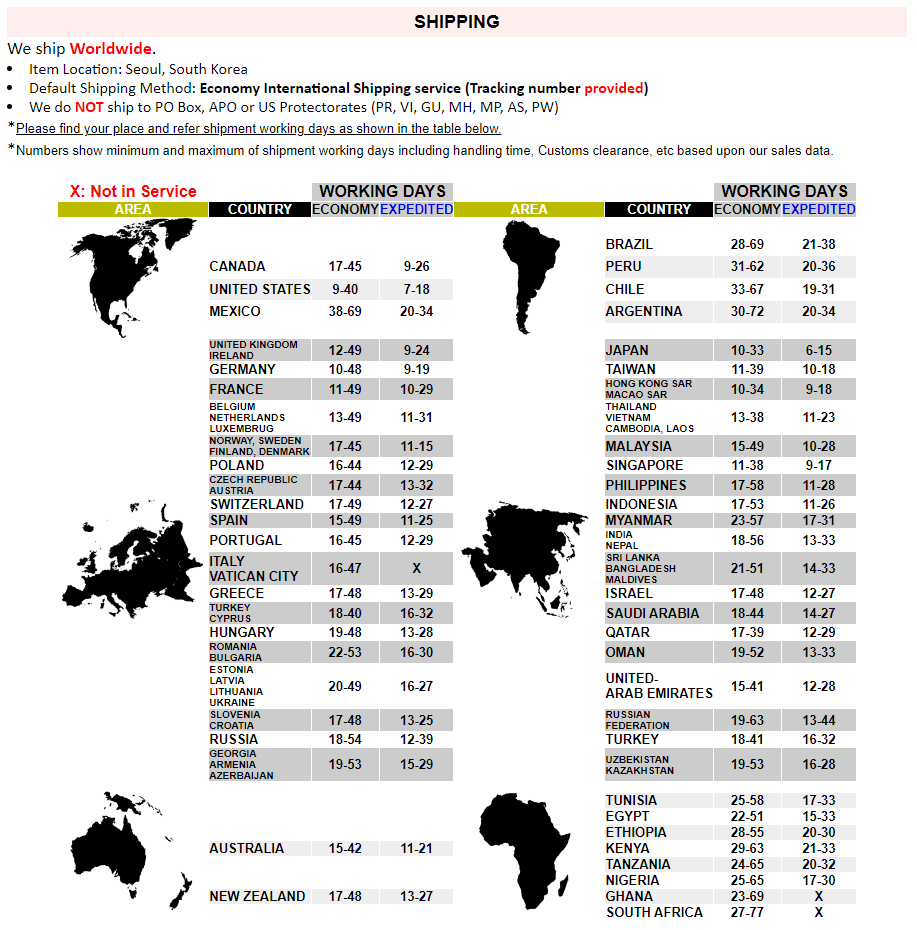 |
|
|
|||
 |










